Dr. Anna Justen
Contact
Telephone
(+49)231 755-2329
Address
Department of Biochemical and Chemical Engineering
Laboratory of Solids Process Engineering
Room G3-4.16
Emil-Figge-Str. 68
44227 Dortmund

Contents
Abstract
One main challenge in formulation development of pharmaceutical applications is the poor aqueous solubility of many drug substances [1]. Formulations of solid dispersions could be shown to be an appropriate strategy to overcome poor solubility and increase the in vitro dissolution behavior [2,3].
Description
The aim of this work is the development of an apparatus, which enables the production of solid dispersions consisting of submicron drug particles embedded in a crystalline carrier matrix.
A spray drying plant, which successfully produces submicron drug particles has been developed. In the custom-made spray drying plant small droplets of API solution are produced with ultrasonic atomization technique. After a drying and a condensation step to remove the solvent from the process, submicron API particles result (Figure 1). In future research the spray drying plant will be optimized concerning the yield and be prepared for further processing of the product.
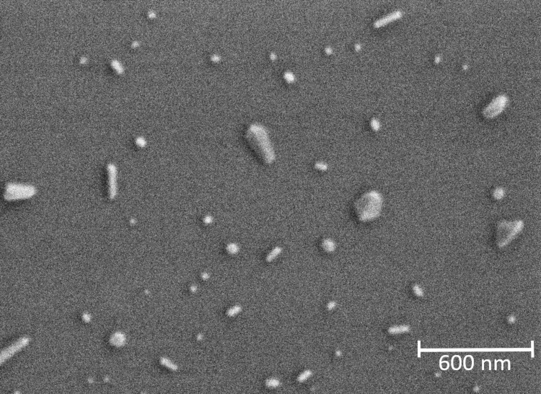
For the embedding of submicron drug particles in a crystalline carrier, electrostatic precipitation technique was found to be an appropriate method. First experiments with a static melt electrostatic precipitator have been conducted [4]. Hereby the concept of the spray drying plant combined with electrostatic precipitation in order to produce solid dispersions could be proved. An experimental set-up has been constructed (Figure 2).
Since the drug load was found to be rather small with this plant, a new concept of an electrostatic precipitator will be developed in future research. A main focus will be the dynamic renewing of the melt surface, so that drug particle agglomeration will be avoided and the drug load of the product can be increased.
Acknoledgements
This project was made possible by the funding of the European Union and the state of North Rhine Westphalia.
References
- Lipinski, C.A. Drug-like properties and the causes of poor solubility and poor permeability. J Pharmacol Toxicol 2000, 44, 235-249.
- Van den Mooter, G. The use of amorphous solid dispersions: A formulation strategy to overcome poor solubility and dissolution rate. Drug Discovery Today: Technologies 2012, 9, e79-e85.
- Thommes, M.; Ely, D.R.; Carvajal, M.T.; Pinal, R. Improvement of the dissolution rate of poorly soluble drugs by solid crystal suspensions. Mol Pharm 2011, 8, 727-735.
- Dobrowolski, A.; Pieloth, D.; Wiggers, H.; Thommes, M. Electrostatic precipitation of submicron particles in a molten carrier. Pharmaceutics 2019, 11.
This project was funded by the European Regional Development Fund (EFRE).
www.efre.nrw.de
www.wirtschaft.nrw.de
Curriculum Vitae
| Since 2019 | PhD at the Laboratory of Solids Process Engineering, TU Dortmund |
| 2018 | Licensed as Pharmacist |
| 2018 | Trainee at Bayer AG, Wuppertal |
| 2017 - 2018 | Trainee at Herz-Apotheke, Bochum |
| 2013 - 2017 | Pharmacy Studies, WWU Münster |
| 2005 - 2013 | Schiller-Schule, Bochum |
| Born | June 1st, Witten (Germany) |



