René Brands
Contact
Telephone
(+49)231 755-2838
Address
Department of Biochemical and Chemical Engineering
Laboratory of Solids Process Engineering
Room G3-4.18
Emil-Figge-Str. 68
44227 Dortmund



Contents
Abstract
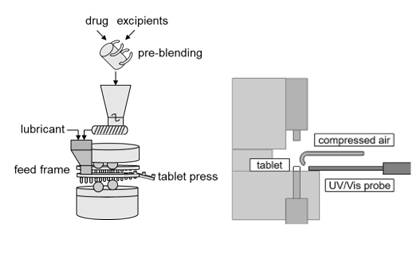
Dry granulation on a rotary tablet press transfers powder materials, consisting of active pharmaceutical ingredients (API) and excipients (lubricants, fillers, binders) into tablets. This process can be divided into sub-processes: feeding, bleeding, filling, compression and ejection. All established control methods for tableting depend on human intervention, which is proven to errors. Therefore, in this project, the human intervention will be replaced by autonomous control for process start up and production phase. In general, autonomous process control requires real-time monitoring of current product quality in order to adjust process parameters online.
Description
Especially spectroscopic methods are suitable for this purpose, since they are fast and non-destructive. Therefore, a UV/Vis based in-line real-time monitoring for tablet API weight fraction, density/porosity and hardness is developed in this project.
Since the tablet quality and the ejection force are dependent on magnitude of lubrication, the lubrication is optimized with focus on reducing shear forces and influence on tablet properties.
References
- Su, Q., S. Ganesh, M. Moreno, Y. Bommireddy, M. Gonzalez, G.V. Reklaitis, and Z.K. Nagy. 2019. “A perspective on Quality-by-Control (QbC) in pharmaceutical continuous manufacturing.” Computers & Chemical Engineering 125:216–31.
- Singh, R., M. Ierapetritou, and R. Ramachandran. 2012. “An engineering study on the enhanced control and operation of continuous manufacturing of pharmaceutical tablets via roller compaction.” International Journal of Pharmaceutics 438(1):307–26.
- Jivraj, M., L.G. Martini, and C.M. Thomson. 2000. “An overview of the different excipients useful for the direct compression of tablets.” Pharmaceutical Science & Technology Today 3(2):58–63.
- Sándor Görög, and S. Görög. 1995 // 2018. Ultraviolet-Visible Spectrophotometry in Pharmaceutical Analysis // Ultraviolet-visible spectrophotometry in pharmaceutical analysis. CRC Revivals. CRC Press: CRC Press - Taylor & Francis Group.
- Backere, C. de, T. de Beer, C. Vervaet, and V. Vanhoorne. 2020. “Evaluation of an external lubrication system implemented in a compaction simulator.” International Journal of Pharmaceutics 587:119675
Curriculum Vitae
| Since 2020 | PhD at the Laboratory of Solids Process Engineering, TU Dortmund |
| 2021 | Licensed as Pharmacist |
| 2020 | Trainee at Bären Apotheke, Bottrop |
| 2019 | Trainee at NextPharma, Waltrop |
| 2015 - 2019 | Pharmacy Studies, HHU Düsseldorf |
| Born | July 16th, Borken (Germany) |

